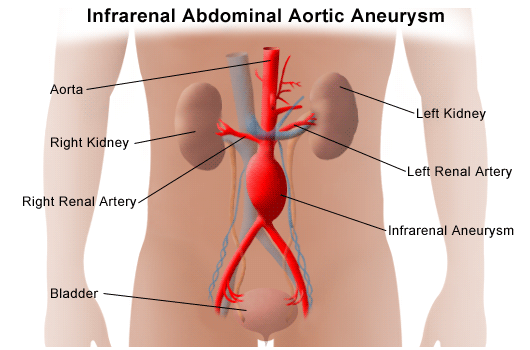
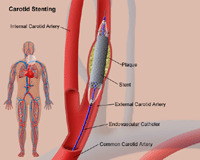
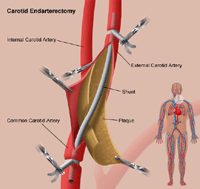
Renal Artery Disease
VIVA III: FORTRESS
A non-randomized study to evaluate the safety and filter efficiency of the Lumen Biomedical FiberNet Embolic Protection System when used in conjunction with the FDA-approved Express SD stent system (Boston Scientific) for stenting of ostial atherosclerotic renal lesions.
HERCULES
A non-randomized study to assess the safety and efficacy of the RX Herculink Elite Renal Stent System (Abbott) for the treatment of suboptimal post-procedural percutaneous transluminam angioplasty (PTA) in atherosclerotic de novo or restenotic renal artery stenosis in patients with uncontrolled hypertension.
Lower-Extremity Peripheral Vascular Disease
VIVA I: XCELL
A non-randomized study to evaluate the safety and performance of the Expert stent (Abbott) in below-the-knee lesions as part of an overall treatment for infrapopliteal lesions in patients undergoing percutaneous intervention for critical limb ischemia.
VIVA II: SALVAGE
A non-randomized study to evaluate the safety and performance of Spectranetics LASER used with Viabahn Endoprosthesis with Heparin Bioactive Surface (Gore) for the treatment of superficial femoral artery in-stent restenosis.
iCAST
A non-randomized study to evaluate the non-inferiority of the iCAST covered stent (Atrium Medical) for the treatment of iliac artery stenoses in patients with de novo or restenotic lesions in the common and/or external iliac arteries.
DURABILITY
A non-randomized study to evaluate the use of the PROTEGE EverFlex Nitinol stent system (ev3) in lesions of the superficial femoral artery and proximal popliteal artery.
ZILVER PTX
A randomized study to compare the long-term safety and effectiveness of the Zilver PTX Drug-Eluting Vascular Stent (Cook Medical) to percutaneous transluminal angioplasty control in patients with de novo or restenotic lesions of the above-the-knee femoropopliteal artery that have not been previously stented.
Device-Based Therapy for Congestive Heart Failure
Promote Q CRT-D and Quartet LV Lead
A multi-center non-randomized study to assess the safety and efficacy of the new multi-polar left ventricular (LV) lead (St. Jude Medical) in the performance of cardiac resynchronization therapy defibrillator (CRT-D) in patients with impaired left ventricular systolic function and congestive heart failure
OPTIMIZE CRTD
A multi-center randomized study to assess the safety and efficacy of the automated optimization of pacing timing using the QuickOpt (St. Jude Medical) algorithm as compared to other methods.
Advanced Cardiovascular Specialists
2490 Hospital Drive, Suite 311 Mountain View, CA 94040 (650) 962-4690